- 06.0Cover
- 06.1Whose Woods These AreZackery Hobler
- 06.2How to Read this Broadsheet
- 06.3An Open Letter to Extinction RebellionWretched of the Earth
- 06.4Forging the Land on PaperBonnie Devine
- 06.5Rag CosmologyErin Robinsong
- 06.6Golden FuturesOrit Halpern
- 06.7for the watersAlize Zorlutuna
- 06.8The Collective Afterlife of ThingsSarah Pereux, John Paul Ricco
- 06.9Closing the Carbon Loop with Artificial PhotosynthesisPhil De Luna
- 06.10A Dictionary for the Future PresentThe Bureau of Linguistical Reality
- 06.11ExtractionsThirza Cuthand
- 06.12To Be RepairedJoy Xiang
- 06.13Demon Copper
Michael DiRisio
- 06.14What is Value?D.T. Cochrane
- 06.15The Blue Dot MovementCiara Weber
- 06.16Local Useful Knowledge: Resources, Research, Initiatives
- 06.17Glossary
Closing the Carbon Loop with Artificial Photosynthesis
- Phil De Luna
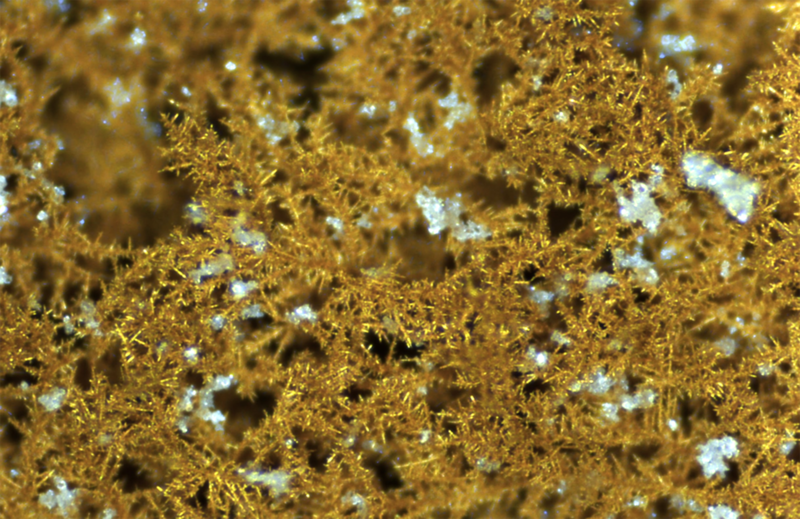
Human activity on the Earth is indisputably demanding more and more energy. As the global population increases rapidly, and quality-of-life measures rise in some developing economies, energy demand continues to grow. Although technological advances can promote longer, fuller, and more productive lives, the corresponding demands on energy production pose a challenge to the urgency of sustainable development.
Energy production fuels global growth and prosperity, but worldwide, the majority of energy continues to be generated from fossil fuel combustion, which contributes to climate change through the production of carbon dioxide (CO2) emissions. The increase in the atmospheric concentration of CO2 is causing our world to warm, alongside numerous related and adjacent effects. Extreme weather events are increasing, biodiversity is decreasing, and all across the globe people’s everyday lives are being disrupted or uprooted by the changing climate.
Thankfully, renewable energy sources such as solar, wind, hydro, and geothermal can mitigate CO2 concentrations by generating emissions-free electricity. Fossil fuels, after all, are simply liquefied and pressured sunlight that has accumulated over millions of years. Renewable energy simply cuts out the middle step: the growth of plants and organic matter that die, decompose, accumulate, and liquefy underground over millennia.
Why, then, aren’t we using solar panels everywhere? If there’s more than enough energy provided by the sun, why aren’t we using it more? As with most things in our world, it all boils down to cost. Photovoltaics have seen massive cost decreases in recent years, with some jurisdictions even pricing solar electricity cheaper than coal.11International Renewable Energy Agency, “Renewable Power Generation Costs in 2018,” May 2019, https://www.irena.org/publications/2019/May/Renewable-power-generation-costs-in-2018. Solar power is particularly affordable in areas with a lot of sunlight, but conversely, it is more expensive in areas that see inconsistent sunlight (such as much of Canada). The mismatch between solar electricity generation in the middle of the day, and the demand to power your TV to watch Netflix at night, is the largest barrier to fully renewable electricity grids. Intermittency spans not only night and day, but also months and seasons. We won’t be heating homes in the winter with solar electricity alone any time soon, nor will we be flying airplanes or moving freight with batteries and solar cells. Renewable energy sources still have much lower energy density (or the amount of energy generated per a given weight or volume) than liquid fossil fuels.

Beyond just energy production, nearly all everyday goods are somehow implicated in fossil fuel production. From the carbon in your phone’s plastic components, to the synthetic fibres in your shirt, to the fertilizers used in industrial food production—all of these goods are linked to processes that emit high amounts of CO2. The comfortable lives many people enjoy is only possible because of our addiction to fossil fuels.
There is another way. Nature’s elegant solution is photosynthesis, which synthesizes water, sunlight, and CO2 into energy, in the form of sugars. However, photosynthesis is less than one percent efficient, and it takes years to grow a tree. By comparison, solar cells are now twenty-two percent efficient in turning sunlight to electricity.22National Renewable Energy Library, “Best Research-Cell Efficiency Chart,” 2019, https://www.nrel.gov/pv/cell-efficiency.html. The amount of CO2 emitted in the atmosphere annually through human activity is vastly larger than what photosynthesis can sequester alone.
In research labs around the world, scientists and engineers are working on a technology called artificial photosynthesis. This process replicates what nature does: it combines energy, CO2, and water, and then engineers that process to make chemicals and fuels. For example, CO2 can be electrochemically converted into a variety of hydrocarbons such as ethylene (the precursor to every major consumer plastic), methane (natural gas), and ethanol (a fuel). In doing so, artificial photosynthesis generates usable materials from air pollution. The idea is simple: can we make fuels and chemicals, the building blocks of our society, from the CO2 in the air rather than from crude oil in the ground? The successful deployment of this process would allow us to convert excess renewable energy into a stable chemical form (as fuels) for long-term seasonal energy storage, thereby solving the problem of intermittency. In addition, fuels made from artificial photosynthesis could integrate into existing and costly energy infrastructure—such as pipelines, tankers, and even your car’s gas tank. This is the idea of a truly circular economy, of engineering the carbon cycle, of working towards a renewably powered future.
There are two steps to artificial photosynthesis: capturing CO2 from the air, and then converting it into something useful. Carbon capture technologies, which address the first step, are beginning to enter the market. Vancouver-based company Carbon Engineering is one leader in this emerging technology. My PhD research was focused on the second step of artificial photosynthesis: converting CO2 into something useful. I led a team to the finals of the Carbon XPRIZE, a $20M competition which challenges entrants to capture CO2 and convert it into a valuable good. Our team, Carbon Electrocatalytic Energy Toronto, focused on generating carbon-based fuels, and base chemicals used in the production of everyday goods.
Artificial photosynthesis is an energy-intensive process. The energy sources powering this process need to be renewable in order for it to close the carbon cycle. At the heart of this technology is a catalyst—a material that combines renewable electricity and CO2, transforming them into fuels and chemicals. Catalysts come in a variety of materials, like copper, silver, or gold, and are synthesized and engineered in the lab. Catalysts lower the energy required for the electrosynthetic reaction to occur—they accelerate the process by making it more efficient. Although research is ongoing, catalyst materials are not yet at the point of industrial commercialization. Stability issues (or how long the catalyst can last before being deactivated) continue to pose challenges to researchers. The issue of selectivity, or being able to control which exact fuel or chemical you make from CO2 (and to make as pure of an output stream as possible), is also a hurdle to overcome.
These are the technical problems that I will be tackling with the Materials for Clean Fuels Challenge Program at the National Research Council. We are committing $60M over seven years to develop technology that can produce zero-emissions transportation fuels and decarbonize industrial processes. This program is building on my previous collaborative research at the University of Toronto by scaling these technologies from the lab bench into larger devices and systems. This endeavour is just beginning, but the time is right—and urgent—for Canada to mitigate the environmental effects of the petrochemical, oil, and gas sectors by becoming a global leader in these technologies.
See Connections ⤴